Oceanite
Category: Volcanic
Type Picritic basalt.
Commons Oceanite is a variety of melanocratic picritic basalt with abundant olivine phenocrysts (20 – 50 vol. %) and less frequent augite phenocrysts. The groundmass is composed of augite, olivine, and plagioclase.
Name origin Oceanite was described for the first time from the Piton de la Fournaise shield volcano on the eastern side of the Réunion Island in Indian Ocean by Lacroix in 1923. This volcano recently belongs to the most active volcanoes of the Earth. It is a part of the Réunion National Park registered in the World´s Cultural and Natural Heritage of the UNESCO. Antoine Lacroix used the term oceanite only for a picritic basalt with more than 50 vol. % of olivine phenocrysts. Today, the term is also used for other picritic basalts with a lesser amount of the phenocrysts.
Locality Mauna Loa, western rift, eruption 1968 (sample courtesy of J. Lexa).
GPS: 19° 28' 12" N, 155° 34' 48" W
Major minerals Olivine and augite in form of phenocrysts. Groundmass is composed of augite, olivine and plagioclase. Olivine phenocrysts are normally zoned, with decreasing MgO and increasing FeO from core to rims. Megacryst cores are the most basic (Fo87-90), phenocrysts 2 – 2.5 mm in size are less basic (Fo83-84), and the groundmass olivine exhibits the widest extent of the chemical composition (Fo81-61) (Wilkinson & Hensel, 1988). The groundmass augite is more depleted in Ca in comparison with augite phenocrysts.
Accessory minerals Magnesiochromite and chromite enclosed in olivine phenocrysts. Ti-magnetite and ilmenite in the groundmass.
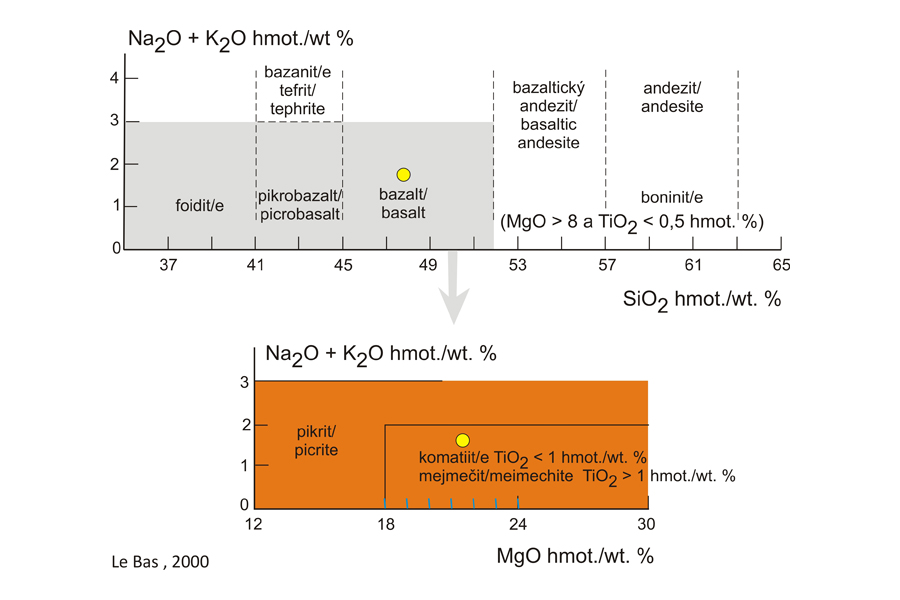
Classification The rock is classified according to SiO2 and Na2O + K2O contents in the TAS diagram (Le Bas et al., 1986). If the chemical composition projects in the shaded field and the rock has an increased MgO content, it corresponds to the picritic basalt. Picrite is a high-Mg rock defined strictly by the following chemical composition: SiO2 30-50 wt. %, MgO > 12 wt. % and Na2O+K2O < 3 wt. %. Within these compositional limits, picrites may fall in foidite, picrobasalt or basalt fields. Oceanite projects in the basalt field (yellow point). The MgO content of the picritic basalt corresponds to 21.48 wt. % (see Table of chemical composition, sample ML-1868). Komatiite and meimechite are ultramafic volcanic rocks with Na2O and K2O contents lower than those in some oceanites. Komatiite differs from the oceanite by typical spinifex structure and a low TiO2 content. Meimechite always contains glass in its groundmass, whereas oceanite only rarely contains glassy matrix.

Colour Black, with yellow-green olivine phenocrysts.
Structure Vesicular, porous.
Granularity Finely grained matrix (0.1 – 1 mm) with olivine phenocrysts, up to 5 mm in size.
Texture Porphyric.
Alterations The rock is unaltered.
Petrographic characteristics Oceanite is porphyric picritic basalt with conspicuous olivine phenocrysts. The phenocrysts may be in some samples larger than 8 mm in diameter (Wilkinson & Hensel, 1988). Phenocrysts larger than 3 mm are called megacrysts. However, an average size of the phenocrysts is between 2 and 2.5 mm, and the groundmass olivine is smaller than 1 mm. The rock is vesicular and contains numerous empty cavities remaining after liberation of gas. The cavities are not filled by secondary minerals.
Usage In foundries and boiler houses as an insulator, because olivine is refractory mineral with a high temperature of melting (~1200 °C).
Literature Clocchiatti, R., Havette, A. & Nativel, P., 1979: Petrogenetic relations between transitional basalts and oceanites from trapped melts in olivine and chromespinel phenocrysts of Piton de la Fournaise (Reunion Island, Indian ocean). Bull. Mineral., 102, 511-525. Cohen. A.S, O´nions, R.K. & Kurz, M.D., 1996: Chemical and isotopic variations in Mauna Loa tholeiites. Earth Planetary Science Letters, 143, 111-124. Le Bas, M. J., 2000: IUGS Reclassification of the High-Mg and Picritic Volcanic Rocks. Journal of Petrology, 41, 1467-1470. MacDonald, G.A. & Katsura, T., 1961: Variations in the lava of the 1959 eruption in Kilauea Iki. Pacific Sci., 15, 358-369. Norman, M.D. & Garcia, M.O, 1999: Primitive magmas and source characteristics of the Hawaiian plume: Petrology and geochemistry of shield picrites. Earth Planetary Science Letters, 168, 27-44. Rhodes, J.M., 1995: The 1852 and 1868 Mauna Loa picrite eruptions: Clues to paental magma compositons and the magmatic plumbing system. In: Rhodes, J.M. & Lockwood: Mauna Loa Revealed: Structure, composition, history and hazards. American Geophysical Union,Washington, D.C., 241-262. Sobolev, A.V. & Nikogosian, I.K, 1994: Petrology of long-lived mantle plume magmatism: Hawaii, Pacific, and Reunion Island, Indian ocean. Petrology, 2, 111-144. Welsch, B., Faure, F., Famin, V., Baronnet, A. & Bachelery, P., 2013: Dendritic crystallization: A single process for all the textures of olivine in basalts? Journal of Petrology, 54, 539-574. Wilkinson, J.F.G., & Hensel, H. D., 1988: The petrology of some picrites from Mauna Loa and Kilauea volcanoes, Hawaii. Contribution to Mineralogy and Petrology, 98, 326–345.
Photomicrographs
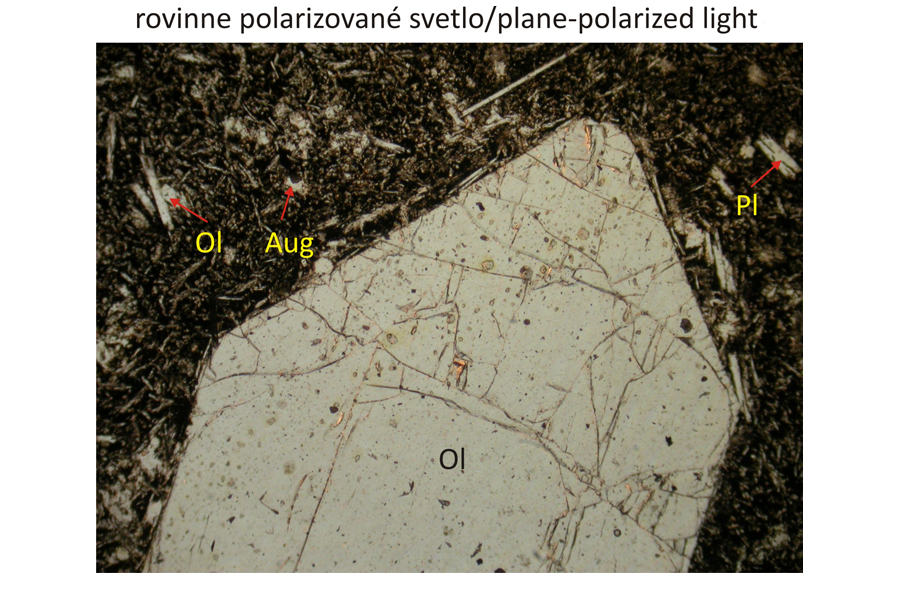
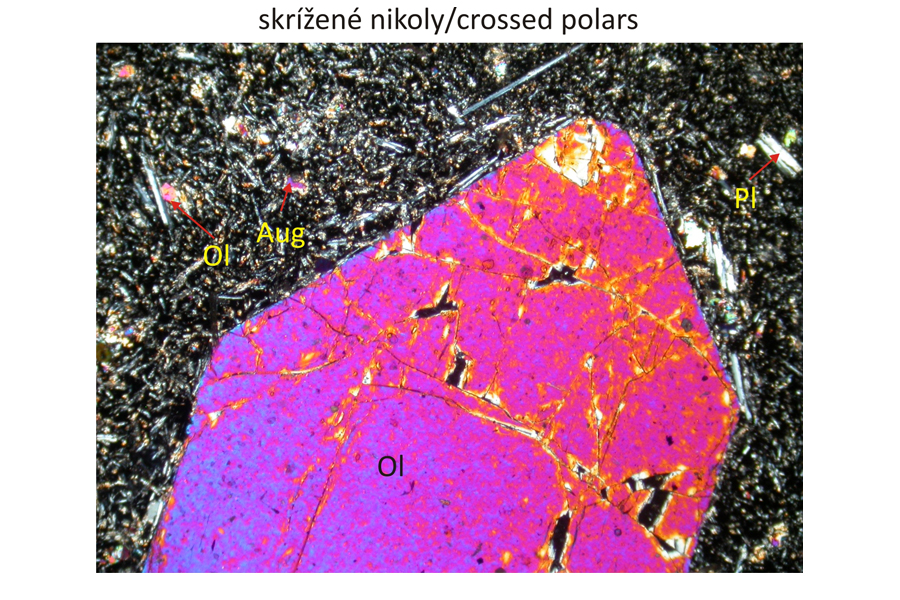
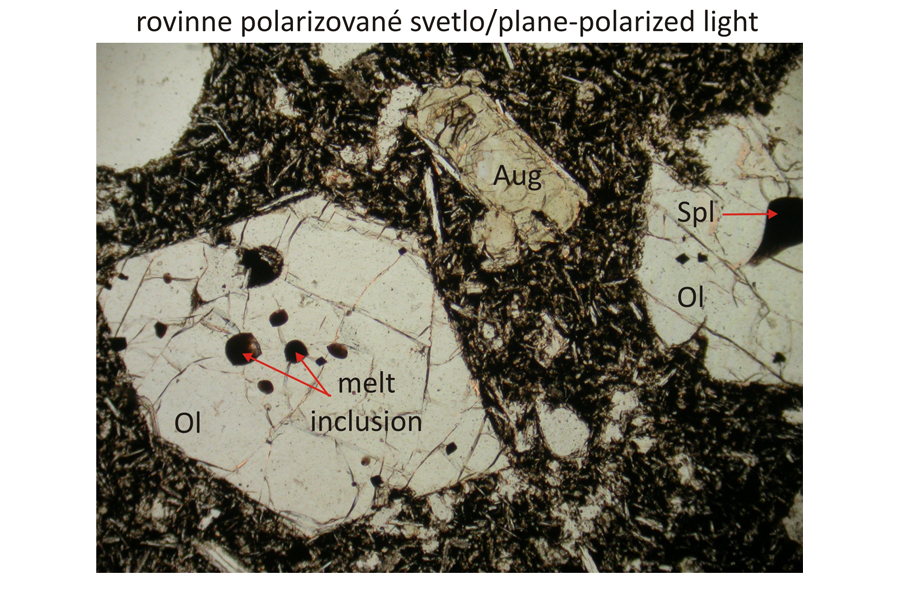
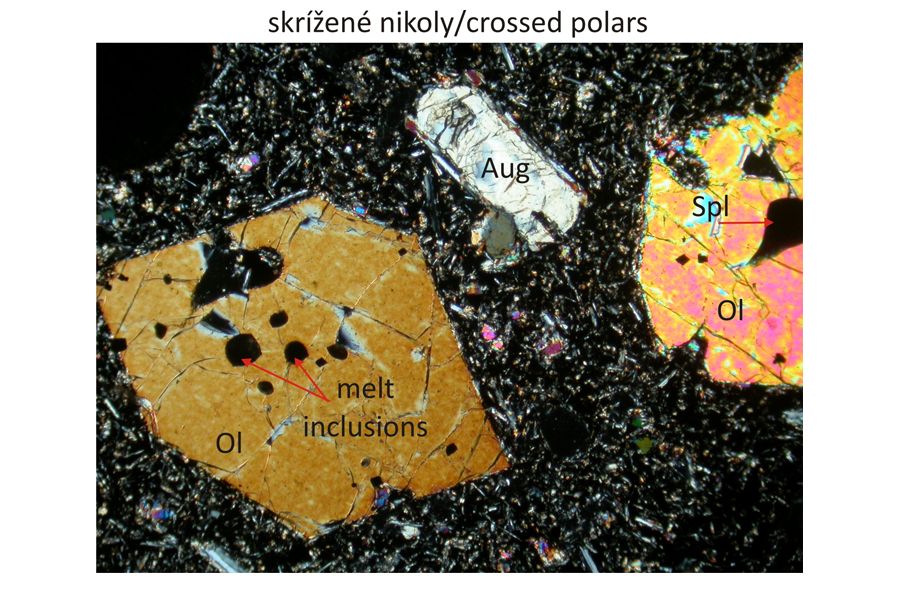
Euhedral phenocrysts of olivine – Ol and augite – Aug in finely grained groundmass composed of olivine, plagioclase – Pl, and augite. Spinel – Spl, corresponding to magnesiochromite or chromite, occurs as inclusions in olivine phenocrysts The olivine phenocrysts also contain inclusions of silicate melts, which are brown in plan-parallel polarized light and exctinct (black) in crossed polars, because they are amorphous. Width of photomicrographs corresponds to 2.2 mm.
Normative composition
Oceanite is hypersthene – hy and olivine – ol normative tholeiitic basalt. Low Na2O and K2O contents result in low contents of normative albite – ab and orthoclase – or.
Normative minerals
SiO2
TiO2
ZrO2
Al2O3
Fe2O3
FeO
MnO
MgO
CaO
Na2O
K2O
P2O5
F
S
CO2
Total
Molar proportion of normative mineral
Molecular mass of normative mineral
Weight % of normative mineral
Oxide
(wt. %)
47.96
1.37
8.88
2.29
9.27
0.18
21.48
6.82
1.46
0.26
0.14
0.01
100.12
Molecular
weight
60.08
79.88
101.96
159.69
71.85
70.94
40.31
56.08
61.98
94.20
141.95
44.01
Molecular
proportion
0.7983
0.0172
0.0871
0.0143
0.1316
0.0025
0.5329
0.1216
0.0236
0.0028
0.0010
0.0002
ap
0.0033
0.0010
0.0010
328.68
0.32
il
0.0172
0.0172
0.0172
100.09
2.60
cc
0.0002
0.0002
0.0002
100.09
0.02
or
0.0166
0.0028
0.0028
0.0028
556.67
1.54
ab
0.1413
0.0236
0.0236
0.0236
278.21
16.91
mt
0.0143
0.0143
0.0143
231.54
3.32
zvyšky
0.1001
0.5329
di
0.1146
0.0091
0.0483
0.0573
0.0573
221.55
12.70
hy´
0.5756
0.0910
0.4846
0.5756
105.38
60.66
ol
0.1714
0.0271
0.1442
0.1714
150.66
25.83
hy
0.2327
0.0639
0.3403
0.2327
105.38
24.53
D: 0.1714
Mg/(Mg+Fe2+): 0.842
Total of normative wt. % 100.12
Comment The CO2 content projects in normative calcite – cc. A portion of provisional hypersthene – hy´ was replaced by true hypersthene – hy and normative olivine – ol.
Chemical composition
Oceanite is a rare high-Mg volcanic rock, which corresponds to sub-alkalic olivine tholeiitic basalt. Hgh MgO (17.7-22.2 wt. %), and low Na2O and K2O contents (0.5 – 2.10 wt. %) are diagnostic for the oceanite. Oceanite is also typical of increased contents of some compatible elements, such as Ni and Cr, proving a primitive character of the magma. The parental magma of oceanite originates at an increased degree (35 – 40 %) of partial melting of spinel lherzolite relatively enriched in Fe (with a Mg-number Mg # = Mg/(Mg+Fe2+ = 0.84). Picritic basalts do not crystallize directly from the melt enriched in Mg (20 – 25 % MgO). In turn, they originate by the accumulation of olivine phenocrysts in a tholeiitic melt depleted in Mg, which represents a residuum after crystallization of phenocrystic olivines from a parental melt with 12-14 wt. % MgO (Wikinson & Hensel, 1988). Oceanite from the 1868 eruption of Mauna Loa contains 864–1439 ppm Ni and 959-1451 ppm Cr (Rhodes, 1952; Wilkinson & Hensel, 1988; Norman & Garcia, 1999; Cohen et al., 1996; Sobolev & Nikogosian, 1994). Oceanites are known mainly from the 1848 eruption of the shield volcano of Mauna Loa (Hawai Island) and from later eruptions of the Kilauea shield volcano taking place in the years 1959-1960. They also occur in recent eruptions of the shield volcano of Piton de la Fournaise of the Réunion Island, where the last eruption was recorded in June 2014 (http://www.volcano.si.edu/volcano.cfm?vn=233020).
-
SiO2
47.96TiO2
1.37Al2O3
8.88Fe2O3
2.29FeO
9.27MnO
0.18MgO
21.48CaO
6.82Na2O
1.46K2O
0.26P2O5
0.14H2O-
0.02CO2
0.01F
0.02Cl
0.01Total
100.17Mg(Mg/Fe2+)
0.81
Wright, 1971; Norman & Garcia, 1999.
-
SiO2
46.9TiO2
1.3Al2O3
8.29FeO
11.31MnO
0.16MgO
22.21CaO
6.58Na2O
0.25K2O
0.25P2O5
0.15Total
97.40Mg(Mg/Fe2+)
0.78
Rhodes, 1995; Cohen et al., 1996.
-
SiO2
44.58TiO2
1.73Al2O3
9.26Fe2O3
1.27FeO
10.76MnO
0.18MgO
23.46CaO
7.18Na2O
1.10K2O
0.29P2O5
0.19H2O+
0.10H2O-
0.04Total
100.14Mg(Mg/Fe2+)
0.80
MacDonald &Katsura, 1961.
-
SiO2
43.4TiO2
1.57Al2O3
7.94FeO
13.15MnO
0.06MgO
23.23CaO
6.93Na2O
1.61K2O
0.50P2O5
n.a.Total
98.39Mg(Mg/Fe2+)
0.76
-
SiO2
43.7TiO2
1.48Al2O3
7.64FeO
12.88MnO
0.19MgO
24.52CaO
6.31Na2O
1.49K2O
0.34P2O5
0.18Total
98.73Mg(Mg/Fe2+)
0.77